What Exactly are Mitochondria and 8 Fixes for Dysfunction

It’s been a while since you’ve felt 100% healthy. It’s hard to pinpoint when exactly you noticed that you were feeling less energetic, a little bit foggy, and not your more vibrant self. Your brain isn’t working quite right. Perhaps you’ve got some memory-related challenges or are feeling anxious and depressed without any obvious reason.
You think that maybe if you get back into your workout routine, you’ll regain some of your vigor. After all, a little dose of endorphins can go a long way. But each time you try to get back on the workout wagon, you don’t get the usual flood of feel-good chemicals. Instead, your fatigue gets even worse. Eventually, you begin to feel winded from a single flight of stairs.
What the heck is wrong?
To understand your energy crisis, you should consider how your body produces energy in the first place.
Enter the mitochondria.
Mitochondria are tiny sub-units responsible for producing energy inside our cells; they’re the building blocks of our bodies. We’re going to take a look at what happens when our mitochondria malfunction—and how this could be contributing to your health problems.
How Could Your Mitochondria “Break?”
In order to understand how mitochondria become dysfunctional, you ought to first understand how they function normally.
All human cells (aside from red blood cells) contain mitochondria. Under normal circumstances, the mitochondria take in oxygen and fuel from the food we eat to make ATP, in a process called oxidative phosphorylation.
Stay with me here.
ATP is an energy currency molecule used to power the chemical reactions in our cells to keep our bodies running smoothly. No ATP, bodies don’t run so smoothly.
Organs that require lots of energy, such as the brain, eyes, heart, liver, kidneys, and skeletal muscle have the most mitochondria. If they didn’t, they wouldn’t be able to keep up with the high energy demand of their activities, like thinking, pumping and filtering blood, breaking down toxins, and moving.
Mitochondrial dysfunction occurs as a result of an inherited disorder or interfering environmental factors (or a combination of both), resulting in deficits in ATP production. The deficiency begins on a cellular level, and if it’s not corrected, entire organ systems begin to malfunction.
So how do we end up with an energy deficit? Damaged mitochondrial machinery.
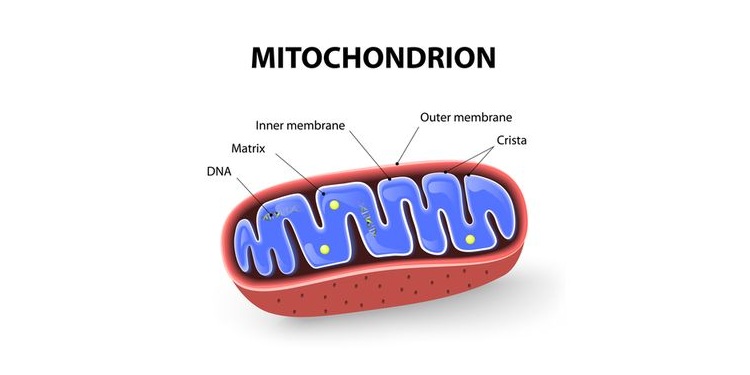
Mitochondria have two membranes—an outer membrane and an inner membrane—both composed of a constellation of proteins and lipids (fats).
ATP production is dependent on the transfer of energy along a sequence of enzymes, referred to as the electron transport chain, that reside in the inner membrane. Again, stay with me here.
Mitochondrial dysfunction can result from damaged membranes or enzymes required for energy transfer along the electron chain. This makes it more difficult to generate enough ATP for your body’s needs.
Low numbers of mitochondria can also contribute to low ATP production. Mitochondria are constantly recycling their parts in order to make more mitochondria that are newer and refreshed in a process called mitochondrial biogenesis. When the mitochondria are dysfunctioning, this process can be affected, which results in lower numbers of mitochondria in our cells.
There are a couple of intricacies in mitochondrial design that predispose them to damage and dysfunction. First, mitochondria contain their own set of DNA (referred to as mtDNA) in the same circular arrangement as bacterial DNA, giving rise to the theory that mitochondria are actually ancient bacteria.
Interestingly, this arrangement of DNA lacks protective histones found in our cellular DNA, making mtDNA particularly susceptible to damage. Damaged mtDNA affects the production of certain mitochondrial proteins, including the enzymes involved in producing ATP.

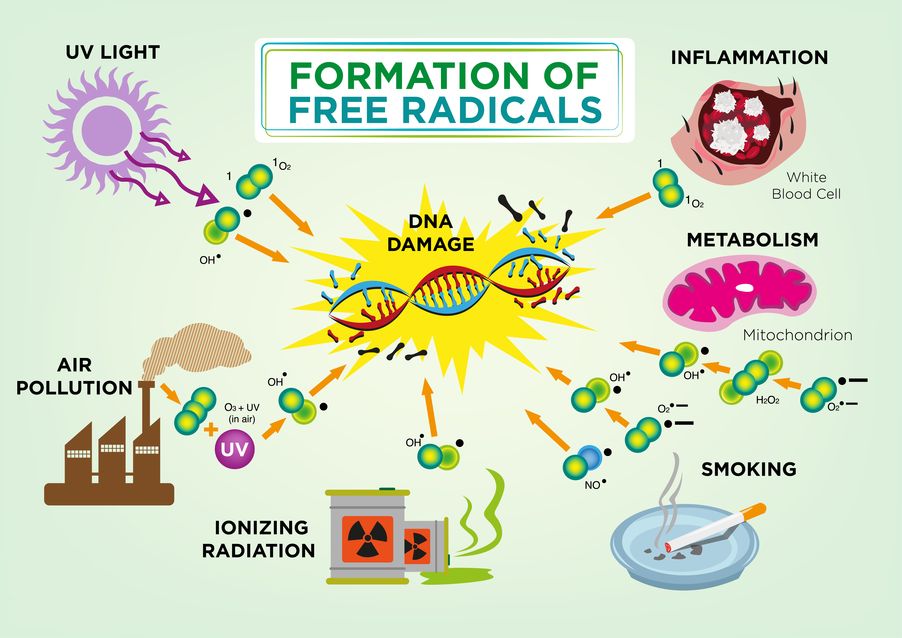
Second, the process of oxidative phosphorylation naturally creates superoxide and hydrogen peroxide, two types of highly reactive oxygen species (ROS). These oxygen-based molecules live inside the mitochondria right next door to their fragile DNA and membranes. ROS react with unprotected mtDNA, as well as the lipids making up the inner and outer membranes. This ROS-driven damage is a type of oxidative stress, since the ROS “oxidize” their victims. As mtDNA is mutated or deleted and the inner membrane is worn down from oxidative stress, the production of ATP may become less efficient.
Mitochondrial damage and dysfunction don’t happen overnight. In fact, it’s been theorized that worn-out mitochondria are a driving force of aging. Our mitochondria have developed systems to mitigate ROS damage, since oxidative phosphorylation is a vital process.
Our cells make enzymes and molecules that are able to deactivate ROS. These include glutathione, catalase, superoxide dismutase, and many others. Antioxidant-rich foods can also counteract the effects of excessive ROS damage by stimulating our natural defense system.
The problems arise when our protective tank is empty but our mitochondrial engines continue to run. Dr. Terry Wahls, MD, an expert in mitochondrial health, outlines the cascade of events leading to this empty antioxidant tank and dysfunctional mitochondria in her book, The Wahls Protocol. Wahls explains how a high toxic load in conjunction with a diet high in refined foods and low in vital nutrients can drain our inherent protective systems and result in an abundance of ROS.

What are The Symptoms of Mitochondrial Dysfunction?
Our mitochondria can only run on fumes for so long before we notice health declines from the accumulating ROS and oxidative stress.
Since mitochondria are present in most cells and the energy they produce is required for most bodily functions, mitochondrial dysfunction doesn’t discriminate when it comes to which systems it affects. Consequently, there are a variety of symptoms corresponding to dysfunctional mitochondria. Some general symptoms are:
Fatigue: Fatigue is one of the most common symptoms of mitochondrial dysfunction. If there’s a large enough energy deficit on a cellular level, you’ll likely experience fatigue.
Lactic acidosis: This is an unusually high amount of lactic acid in your body from a compound called pyruvate, which is usually used up in ATP production.
Environmental illness: You may notice that your symptoms are exacerbated by exposure to toxins in your environment in the form of pesticides, heavy metals, cleaning products, cosmetics, or fungal/bacterial toxins.
Exercise intolerance: Shortness of breath is one aspect, but instead of feeling good after exercise, you may find you’re exhausted and it takes you days to recover.
Dr. Richard Horowitz, MD, author of Why Can’t I Get Better? Solving the Mystery of Lyme and Chronic Disease, presents an impressive list of organ-specific symptoms that can be attributed to dysfunctional mitochondria. Here are some that Horowitz mentions:
Skeletal muscles: Low muscle tone, myofascial pain (pain in the muscle’s soft tissue), and exercise intolerance.
Heart: Cardiac conditions such as cardiovascular disease, enlargement of the heart (cardiomyopathy), heart failure, and atherosclerosis (a build-up of calcium in the arteries, forming clogs).
Liver: Impaired gluconeogenesis (breakdown of stored sugar) leading to low blood sugar; non-alcoholic fatty liver, cancer, and hepatitis.
Kidneys: Imbalances in essential amino acids, electrolytes, and minerals
Metabolism: Metabolic syndrome, which is characterized by a combination of at least three of the following: hypertension, truncal obesity (belly fat), abnormal glucose tolerance, elevated triglycerides, and low HDL cholesterol (good cholesterol).
Dr. Horowitz also describes the link between mitochondrial dysfunction and the following neurological disorders:
Neurodegenerative diseases including Huntington’s, Alzheimer’s, and Parkinson’s.
Psychological disorders such as schizophrenia, bipolar, and anxiety.
Central/peripheral nervous system symptoms including neuropathies leading to hearing loss, changes in vision, and blood pressure and temperature regulation issues.
Dr. Horowitz doesn’t address autism-spectrum disorders in his book, but there’s research suggesting a link between mitochondrial dysfunction and the development of these disorders.1
Dr. Terry Wahls addresses mitochondrial dysfunction as it relates to autoimmunity, such as in multiple sclerosis (MS). Mitochondrial problems have also been linked to chronic fatigue syndrome (CFS) and fibromyalgia.2,3
According to Wahls, being diagnosed with any type of chronic disease greatly increases your chances of having mitochondrial dysfunction and may contribute to overlapping symptoms.
Types of Mitochondrial Dysfunction
Chris Kresser, an expert in functional medicine, also recognizes the link between chronic disease and mitochondrial dysfunction. Kresser reports that mitochondrial dysfunction can be broken down into two types—primary and secondary mitochondrial dysfunction.
Primary: In primary dysfunction, there’s a mutation or group of mutations in the mitochondrial or nuclear DNA that’s inherited from the parents.
Mutations in mtDNA are exclusively passed from mother to child. Typical symptoms of this type of dysfunction can manifest in childhood and include developmental delays, most notably in the areas requiring the most energy.
Although technically any part of our mitochondria can malfunction, primary dysfunctions only include inherited mutations that specifically affect the electron transport chain of the mitochondria.4
Secondary: Secondary dysfunction is characterized by acquired mitochondrial mutations, resulting from internal ROS production and external sources of oxidative stress throughout your lifetime.
This type of dysfunction also considers factors such as the number and structure of mitochondria as contributing factors to malfunction. Secondary dysfunction is commonly associated with “normal” aging.
Root Causes of Mitochondrial Dysfunction
The bad news is that it’s still unclear whether mitochondrial dysfunction is a result of the chronic diseases it’s associated with, or whether it’s a root cause of the problem in the first place.
The good news is that there’s a lot of evidence showing that some changes in diet and lifestyle can be powerful tools in recharging your mitochondria, regardless of whether the dysfunction is a cause or effect of chronic illness. The disorder is also mainly characterized by an overwhelming amount of oxidative stress, so considering the root causes of excessive oxidative stress will also help in healing your mitochondria. We’ll go over the following root causes of mitochondrial dysfunction:
- Refined foods
- Nutrient deficiencies
- Lack of exercise
- Toxic load

Refined Foods
There are a couple of problems when it comes to using refined foods as a fuel source.
First, they’re high in “energy” in the form of highly refined sugars or fats, but essentially void of nutrients. They force your mitochondria to work without the reward of vitamins and minerals to replenish the tank.
Second, when your mitochondria burn these dense energy sources like processed sugar and flour, they’re not burned efficiently. Inefficiency in ATP production leads to excess ROS formation.
Finally, refined oils are uniquely detrimental since they’re almost always oxidized before you even eat them. Oxidized fats can take the place of healthy fats in the mitochondria’s lipid membranes, where they cause oxidative stress and deformities.
Mark Sisson, a paleo nutrition and fitness expert, recommends ditching sugar if you want to recover your mitochondrial function. Sisson points out that our mitochondria may actually do better using primarily fats as a fuel source, since they’re burned with less ROS production.

Nutrient Deficiency
Filling up on refined food tricks your body into thinking that you’re full. But actually, you’re still starving in terms of micronutrient intake, like fat-soluble vitamins (A, D, K2, E), the B family vitamins, essential minerals (zinc, magnesium, potassium, selenium, chromium, sulfur, iron, manganese, and copper, among others), and polyphenols.
Even if you’re eating healthy, nutrient-dense food, poor digestion can often lead to malabsorption of nutrients that contributes to sluggish mitochondria.
The theory is that when there are shortages in micronutrients, the micronutrients you do introduce are reserved for the immediate demands of your body, like moving, breathing, and “fight or flight” responses. This can start to wear on the mitochondria slowly over time, since you’re continuing to live but aren’t providing optimal conditions for long, healthy mitochondrial life.
There’s a lot of research to suggest that micronutrient deficiency is a driver of many modern diseases, as well as aging, which is correlated with a decline in mitochondrial function.14,15

Lack of Exercise
There’s a growing body of research showing an association between unhealthy mitochondria and a sedentary lifestyle.
Some research attributes the evolution of our species to the fact that we’re able to run long distances, increasing our oxygen intake and ATP production efficiency.16
There are also studies to suggest that exercise induces mitochondrial biogenesis.17,18 If we don’t require our bodies to move, our cells won’t require as many mitochondria. If we don’t move, we’re not taking in as much oxygen, and ROS formation increases.
Sayer Ji of greenmedinfo.com cites multiple studies showing the benefits of exercise on mitochondrial health. Given the right fuel and nutrients, our bodies are programmed to respond to the demand of exercise. As Mark Sisson puts it, the more we challenge our cells with physical activity, the more our mitochondria will maintain and proliferate themselves.
Toxicity
Toxicity may be a root cause for many chronic diseases, but toxins are particularly harmful to the mitochondria. When your body has to deal with a lot of toxins, you’re using up vital B vitamins as well as antioxidants like glutathione.
A deficit in the B vitamins, particularly those that support mitochondrial function, will lead to inefficient ATP production and more ROS.
With toxic chemicals ever-present in air pollution, cosmetics, food, cleaning products, and even our water, it’s relatively easy to build up an unhealthy toxic load.
Heavy metals like lead (used in paint) and mercury (used in fillings) have attracted a lot of attention for being detrimental to your health. They set up camp in the membranes of our mitochondria, where they contribute to oxidative stress. Heavy metals are lipophilic, meaning they’re attracted to fat and don’t like water, so instead of being excreted, they can be taken up by the mitochondria.19

Even if you’ve gotten your mercury fillings removed and only eat organic veggies, you may not be in the clear.
Lara Adler, a holistic nutritionist and expert in environmental toxins, reports that 94% of US drinking water is contaminated by a pesticide called Atrazine. Atrazine contributes to weight gain and insulin resistance due to its direct interference with your mitochondria.
Over-the-counter and prescription medicines may also be contributing to your toxic load. Dr. Wahls presents a long list of classes of drugs that have been shown to drain important cofactors of mitochondrial metabolism (B vitamins and minerals) while also depleting our antioxidant reserves, specifically CoQ10.
For example, cholesterol-lowering drugs (called statins) deplete CoQ10, dramatically affecting mitochondrial function. Other interfering drugs listed in Wahl’s book are antidepressants, anxiety medications, acetaminophen (Tylenol), ibuprofen, certain antibiotics, stomach acid reduction medication, birth control, and diabetic medications (metformin). Although medications may be necessary in some cases, it’s important to understand how they can be toxic to your mitochondria in order minimize these detrimental effects.

8 Fixes for Mitochondrial Dysfunction
Let’s talk about a few things that may be able to give your mitochondria some much-needed rest, regardless of the type or source of your dysfunction. This list isn’t exhaustive, but it’s a very, very good start.
1) Anti-inflammatory diet: Mitochondrial dysfunction is accompanied by excess ROS and substantial oxidative stress. Inflammatory cytokines can also stress the mitochondria.5,6 A good anti-inflammatory diet excludes things like gluten, dairy, and other individual food sensitivities that may be causing systemic inflammation. It also allows the lining of your gastrointestinal tract to heal, minimizing the leakage of toxins into the bloodstream, where they can contribute to mitochondrial damage.

2) More vegetables: Dr. Terry Wahls prescribes 9 cups of vegetables a day, including dark fruits and veggies, sulfur-rich veggies like garlic and cruciferous vegetables, and dark leafy greens. These veggies will help to refill your antioxidant tanks. They also provide building blocks for our inherent antioxidants, along with B vitamins and minerals necessary for mitochondrial function.
3) Glutathione: Glutathione is an antioxidant made by your body to combat ROS and other drivers of oxidative stress. Mitochondrial dysfunction has also been shown to inhibit our production and deplete our stores of glutathione.7
Glutathione can be taken in supplement form, but according to Dave Asprey, a well known “biohacker,” research shows that it’s best absorbed in a liposomal form to ensure that it bypasses digestion in the stomach.
Dr. Terry Wahls and Dr. Mark Hyman recommend eating foods rich in sulfur compounds like garlic, broccoli, and cauliflower, which our bodies can use to make glutathione.
4) Co-enzyme Q10: This is another one of your body’s own natural antioxidants that may run out when your mitochondria aren’t working properly. It’s also an integral player in the electron transport chain, as it catalyzes energy transfer and protects mitochondrial membranes from ROS.8
Interestingly, production of this vital enzyme declines with age, and supplementation may be required to maintain optimal levels according to experts like Dr. Wahls and Dr. Horowitz. Additionally, statins tend to deplete CoQ10, so if you’re on a statin medication, you may want to consider CoQ10 to offset this loss.

5) Resveratrol: This naturally-occurring antioxidant found in the skins of red grapes may be particularly beneficial to the mitochondria. Not only is it an antioxidant, but research suggests that it also promotes mitochondrial biogenesis, yielding healthier and more numerous mitochondria.9
6) Alpha lipoic acid (ALA): ALA is a short-chain fatty acid that your body makes naturally that facilitates the transport of glucose into cells. This helps your mitochondria by preventing high blood sugar, which may contribute to their dysfunction.10 Research on ALA also suggests that it promotes mitochondrial biogenesis and acts as an antioxidant, much like resveratrol.11
7) Acetyl-L-carnitine (ALC): ALC is an amino acid that your mitochondria use to help transport fatty acids, particularly the larger ones, into the mitochondria to be used as energy and to repair damaged membranes. Studies show that ALC may be effective in relieving some of the stress on your mitochondria by facilitating transport of materials through damaged membranes.11,12
8) Lipid replacement therapy (LRT): This therapy addresses the damaged mitochondrial membranes by delivering supplemental phospholipids, the type of lipids making up the membranes.
Dr. Horowitz recommends a product called NT factor, which delivers membrane phospholipids like phosphatidylcholine and phosphatidylserine along with antioxidants and fuel sources for your mitochondria to use. One study used NT factor for LRT in patients with chronic fatigue syndrome, and participants noticed improvements in energy after four weeks of taking the supplement.13
While these suggestions can begin to address the immediate needs of your mitochondria, some things in your life will probably have to change to ensure that they continue working in more desirable conditions. After all, there’s probably an underlying reason your mitochondria are feeling overwhelmed and overworked.
